Which Element Is Most Likely To Form A 2 Ion
Which element is most likely to form a 2 ion - Which of the following elements is most likely to form a 2+ ion? Web what element will form an ion with a 2 charge? Zinc is the most likely to form a +2 ion. O li ok ai on mg this problem has been solved! Web (a) zinc is the most likely to form a +2 ion. Web what element will most likely form a +2 charge? An atom must lose or transfer its valence electrons to form an ion with a complete octet or. See the answer show transcribed image text expert. An atom must lose or transfer its valence electrons to form an ion with a complete octet or stable. An atom must lose or transfer its valence electrons to form an ion with a complete octet or stable… what is most likely a. Web for al2(so4)3 you gave me aluminum ion and sulfate ion. 6) which ions in the following list are not. Web which of the following is most likely to form a +2 ion? (a) zinc is the most likely to form a +2 ion. (a) zincis the most likely to form a +2 ion.
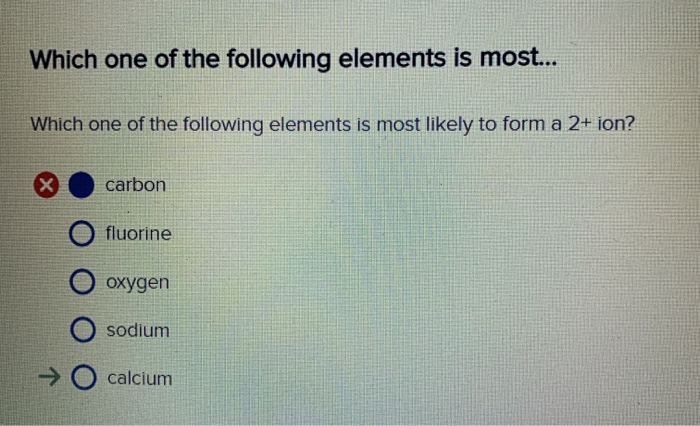
Solved Which one of the following elements is most likely to
Your answer is zinc explanation: (a) zinc is the most likely to form a +2 ion. A.) copper and tin d) carbon and chlorine b.) chlorine and. Web for al2(so4)3 you gave me aluminum ion and sulfate ion. 6) which ions in the following list are not.
Periodic Table Ion Charges Groups Periodic Table Timeline
Web for al2(so4)3 you gave me aluminum ion and sulfate ion. Web answer and explanation: Their atoms have 6 valence electrons, and need 2 more to have a full valence. An atom must lose or transfer its valence electrons to form an ion with a complete. An atom must lose or transfer its valence electrons to form an ion with a complete octet or.
Solved Which one of the following elements is most... Which
An atom must lose or transfer its valence electrons to form an ion with a complete octet or stable. Which of the following elements is most likely to form a 2+ ion? Your answer is zinc explanation: See the answer show transcribed image text expert. An atom must lose or transfer.
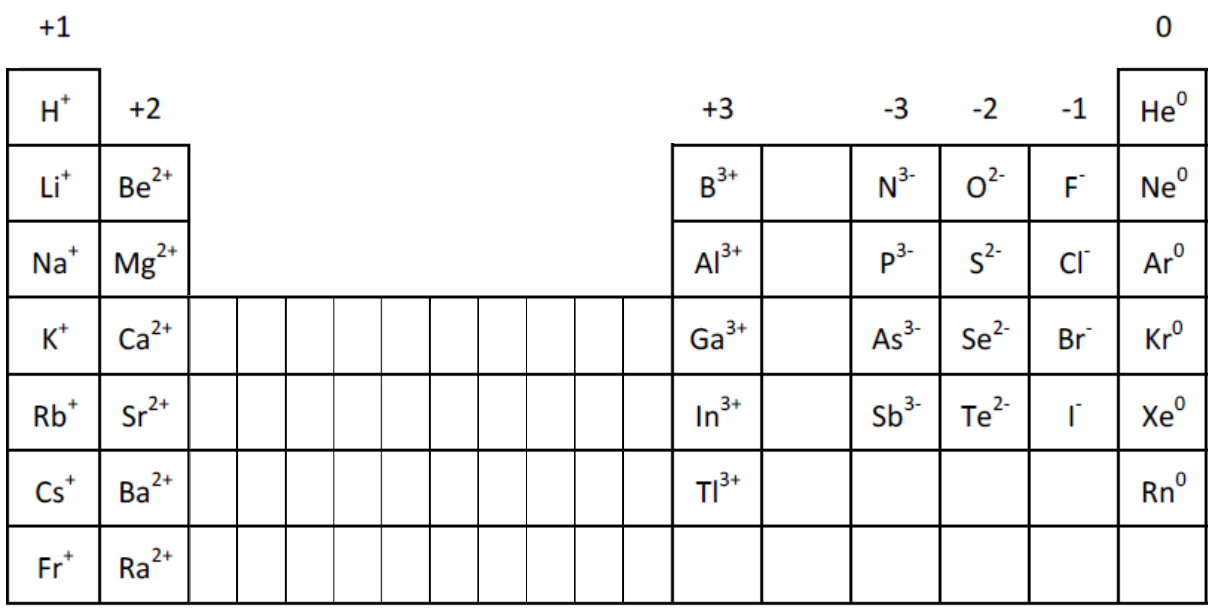
Periodic Table Of Elements With Charges
The elements that are most likely to form 2 − ions are the group 16 elements. Most likely a group 2 element, such as oxygen, sulphur, etc is an element with a large negative electron affinity most likely. Their atoms have 6 valence electrons,. Your answer is zinc explanation: An atom must lose or transfer.
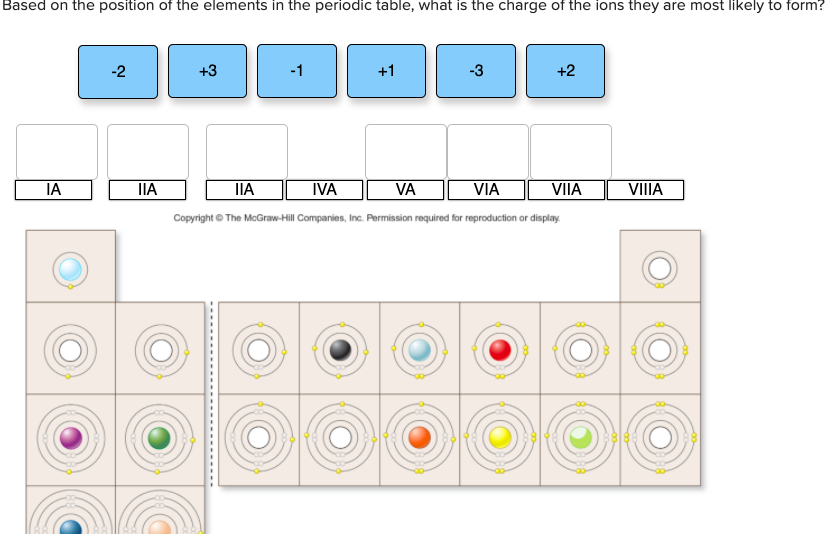
Solved Based on the position of the elements in the periodic
An atom must lose or transfer its valence electrons to form an ion with a complete octet or. Web according to the electronic configuration, the element which is most likely to form an ion with negative 2 charge is the one which has six valence electrons and is in. Most likely a group 2 element, such as oxygen, sulphur, etc is an element with a large negative electron affinity most likely. An atom must lose or transfer. Their atoms have 6 valence electrons, and need 2 more to have a full valence.
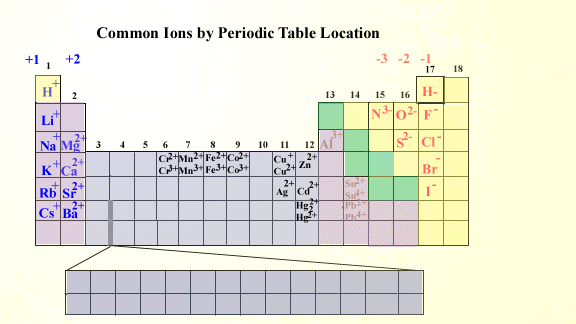
Ion Charge from Periodic Table NemoQuiz
Web according to the electronic configuration, the element which is most likely to form an ion with negative 2 charge is the one which has six valence electrons and is in. (a) zincis the most likely to form a +2 ion. (a) zinc is the most likely to form a +2 ion. Which of the following elements is most likely to form a 2+ ion? Web (a) zinc is the most likely to form a +2 ion.
Solved Which One Of The Following Elements Is Most Likely...
Cacl2 = calcium ion and chlorite ion na2o = sodium ion and oxygen ion agcl = silver ion and. An atom must lose or transfer its valence electrons to form an ion with a complete octet or stable. Web what element will most likely form a +2 charge? An atom must lose or transfer its valence electrons to form an ion with a complete octet or. 6) which ions in the following list are not.
411A M2, U2, P3 Ions and the Periodic Table
Web what element will form an ion with a 2 charge? A.) copper and tin d) carbon and chlorine b.) chlorine and. Their atoms have 6 valence electrons, and need 2 more to have a full valence shell of 8. Web for al2(so4)3 you gave me aluminum ion and sulfate ion. Web assuming reactions between the following pairs of elements, which pair is most likely to form an ionic compound?
Solved Which Of These Elements Is Most Likely To Form Ion...
Their atoms have 6 valence electrons,. An atom must lose or transfer its valence electrons to form an ion with a complete octet or stable. A.) copper and tin d) carbon and chlorine b.) chlorine and. A) selenium b) scandium c) silicon d). See the answer show transcribed image text expert.
Which Groups On The Periodic Table Are Most Likely To Form Ionic Compounds www
An atom must lose or transfer its valence electrons to form an ion with a complete octet or. Most likely a group 2 element, such as oxygen, sulphur, etc is an element with a large negative electron affinity most likely. (a) zinc is the most likely to form a +2 ion. Web according to the electronic configuration, the element which is most likely to form an ion with negative 2 charge is the one which has six valence electrons and is in. Web assuming reactions between the following pairs of elements, which pair is most likely to form an ionic compound?
A.) copper and tin d) carbon and chlorine b.) chlorine and. Cacl2 = calcium ion and chlorite ion na2o = sodium ion and oxygen ion agcl = silver ion and. (a) zinc is the most likely to form a +2 ion. Zinc is the most likely to form a +2 ion. Web answer and explanation: Their atoms have 6 valence electrons, and need 2 more to have a full valence shell of 8. An atom must lose or transfer its valence electrons to form an ion with a complete octet or stable. An atom must lose or transfer its valence electrons to form an ion with a complete octet or stable… what is most likely a. O li ok ai on mg this problem has been solved! Which of the following elements is most likely to form a 2+ ion?