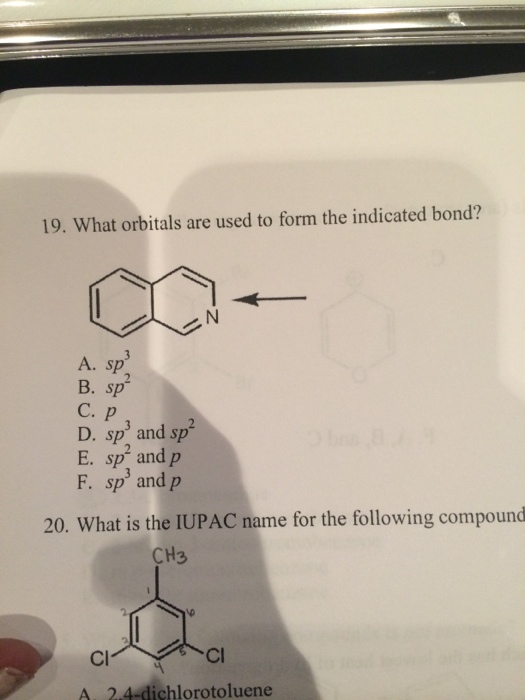
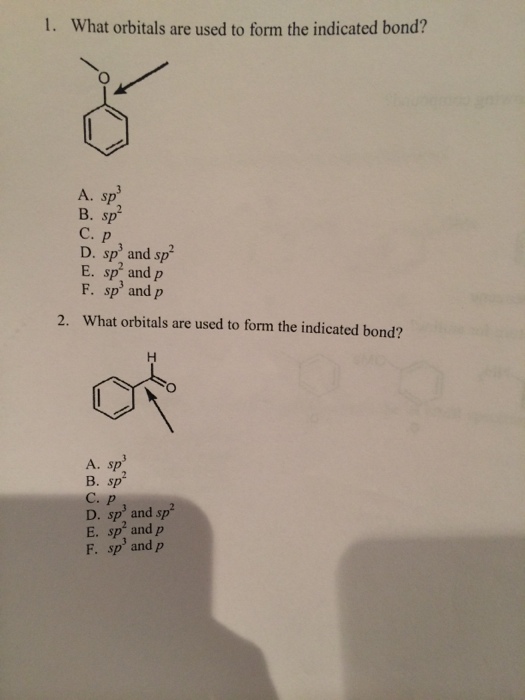What Orbitals Are Used To Form The Indicated Bond
What orbitals are used to form the indicated bond - What orbitals are used to form bonds? Sigma (σ ) and pi (π ). There are two types of overlapping orbitals: Because this carbon is also s p to hybridized for the pie bond pie bonds. Both bonds are formed from the overlap of two orbitals one on each atom. This problem asks you to determine which orbital's are overlapping in order to form a bond, for instance, between carbon and brilliant or between carbon and hydrogen. The signal bond is made up of a carbon s p to orbital overlapping with another carbon s p to orbital. There are two types of overlapping orbitals: Sp 3 and sp 2 e. Sigma (σ ) and pi (π ). What orbitals are used to form bonds? Both bonds are formed from the overlap of two orbitals one on each atom. What orbitals are used to form the indicated bonds?
Solved What orbitals are used to form the indicated bond?
What orbitals are used to form the indicated bonds? What orbitals are used to form bonds? Both bonds are formed from the overlap of two orbitals one on each atom. There are two types of overlapping orbitals: There are two types of overlapping orbitals:
What Orbitals Are Used To Form The Indicated Bond FORM.UDLVIRTUAL.EDU.PE
Sigma (σ ) and pi (π ). There are two types of overlapping orbitals: What orbitals are used to form bonds? Both bonds are formed from the overlap of two orbitals one on each atom. Because this carbon is also s p to hybridized for the pie bond pie bonds.
Solved 4. What orbitals are used to form the indicated bond?
This problem asks you to determine which orbital's are overlapping in order to form a bond, for instance, between carbon and brilliant or between carbon and hydrogen. Both bonds are formed from the overlap of two orbitals one on each atom. What orbitals are used to form bonds? There are two types of overlapping orbitals: There are two types of overlapping orbitals:
What Orbitals Are Used To Form The Indicated Bond FORM.UDLVIRTUAL.EDU.PE
What orbitals are used to form bonds? Sp 3 and sp 2 e. Both bonds are formed from the overlap of two orbitals one on each atom. This problem asks you to determine which orbital's are overlapping in order to form a bond, for instance, between carbon and brilliant or between carbon and hydrogen. The signal bond is made up of a carbon s p to orbital overlapping with another carbon s p to orbital.
Solved Identify the orbitals that are used to form each
What orbitals are used to form bonds? Sigma (σ ) and pi (π ). Both bonds are formed from the overlap of two orbitals one on each atom. There are two types of overlapping orbitals: There are two types of overlapping orbitals:
Solved What orbitals are used to form the indicated bond?
Sigma (σ ) and pi (π ). Because this carbon is also s p to hybridized for the pie bond pie bonds. What orbitals are used to form the indicated bonds? Both bonds are formed from the overlap of two orbitals one on each atom. The signal bond is made up of a carbon s p to orbital overlapping with another carbon s p to orbital.
What Orbitals Are Used To Form The Indicated Bond FORM.UDLVIRTUAL.EDU.PE
Sigma (σ ) and pi (π ). What orbitals are used to form the indicated bonds? Sigma (σ ) and pi (π ). Sp 3 and sp 2 e. This problem asks you to determine which orbital's are overlapping in order to form a bond, for instance, between carbon and brilliant or between carbon and hydrogen.
What Orbitals Are Used to Form the Indicated Bond TrendingWorld
Both bonds are formed from the overlap of two orbitals one on each atom. There are two types of overlapping orbitals: Sigma (σ ) and pi (π ). What orbitals are used to form bonds? Both bonds are formed from the overlap of two orbitals one on each atom.
Solved What orbitals are used to form the indicated bond.
Sp 3 and sp 2 e. Because this carbon is also s p to hybridized for the pie bond pie bonds. Sigma (σ ) and pi (π ). This problem asks you to determine which orbital's are overlapping in order to form a bond, for instance, between carbon and brilliant or between carbon and hydrogen. What orbitals are used to form bonds?
Solved What hybrid orbitals make up the indicated bond in
There are two types of overlapping orbitals: There are two types of overlapping orbitals: Both bonds are formed from the overlap of two orbitals one on each atom. Both bonds are formed from the overlap of two orbitals one on each atom. What orbitals are used to form bonds?
There are two types of overlapping orbitals: What orbitals are used to form the indicated bonds? Sp 3 and sp 2 e. What orbitals are used to form bonds? Sigma (σ ) and pi (π ). This problem asks you to determine which orbital's are overlapping in order to form a bond, for instance, between carbon and brilliant or between carbon and hydrogen. There are two types of overlapping orbitals: The signal bond is made up of a carbon s p to orbital overlapping with another carbon s p to orbital. Sigma (σ ) and pi (π ). Because this carbon is also s p to hybridized for the pie bond pie bonds.