Methanol Ch3oh Forms When Carbon Monoxide Reacts With Hydrogen
Methanol ch3oh forms when carbon monoxide reacts with hydrogen - Question 12 of 15 > carbon monoxide, co, reacts with hydrogen, h, to form methanol according to the following reaction. Select the sample of co gas that will react completely with the sample of h, gas. Carbon monoxide reacts with hydrogen under certain condition to form methanol (ch 3oh). When 0.60 mol of carbon monoxide and 0.40 mole hydrogen gas are allowed to reach equilibrium in a 1.0l container, 0.030 moles of methanol are formed. What is the value of kc? What is reaction condition of co (carbon monoxide) reacts with h2 (hydrogen) ? Report answer to the correct number of significant figures. Carbon monoxide gas reacts with hydrogen gas at elevated temperatures to form methanol (ch3oh) gas. Write a balanced chemical equation for this reaction indicating the physical state of reactants and product, as well as the condition under this reaction, take place. N = m m = 45 32 = 1.41 mol. Carbon monoxide reacts with hydrogen under certain conditions to form methanol (ch3oh). Co (g) + 2 h2 (g) ch,oh () a sample of h2 gas is shown in the image. From the balanced equation we know that it takes 2 moles of h 2 to produce each mole of methanol, so we need 2.82 mol of h 2. Carbon monoxide reacts with hydrogen under certain conditions to form methanol (ch3oh). One c, one o and 4 h on each side.
SOLVEDCarbon monoxide and hydrogen gas react to form methanol gas. If Keq= 10.5, [H2]= 0.933mol
2h 2 + co → ch 3oh. Co (g)+2h2 (g)→ch3oh (g) a 1.75 l reaction vessel, initially at 305 k, contains carbon monoxide. Write a balanced chemical equation for this reaction indicating the physical states. Carbon monoxide gas reacts with hydrogen gas to form methanol via the following reaction: The molar mass of methanol is 12 +4 +16 = 32 gmol−1.
Methanol Ch3oh Forms When Carbon Monoxide Reacts With Hydrogen TrendingWorld
What is the value of kc? Question 12 of 15 previous question next question Write a balanced chemical equation for this reaction indicating the physical state of reactants and product, as well as the condition under this reaction, take place. The ideal environmental conditions for. Select the sample of co gas that will react completely with the sample of h, gas.
Solved Carbon Monoxide Gas Reacts With Hydrogen Gas To Fo...
The molar mass of methanol is 12 +4 +16 = 32 gmol−1. Write a balanced chemical equation for this reaction indicating the. From the balanced equation we know that it takes 2 moles of h 2 to produce each mole of methanol, so we need 2.82 mol of h 2. Question 12 of 15 previous question next question Co (g)+2h2 (g)→ch3oh (g) a 1.75 l reaction vessel, initially at 305 k, contains carbon monoxide.
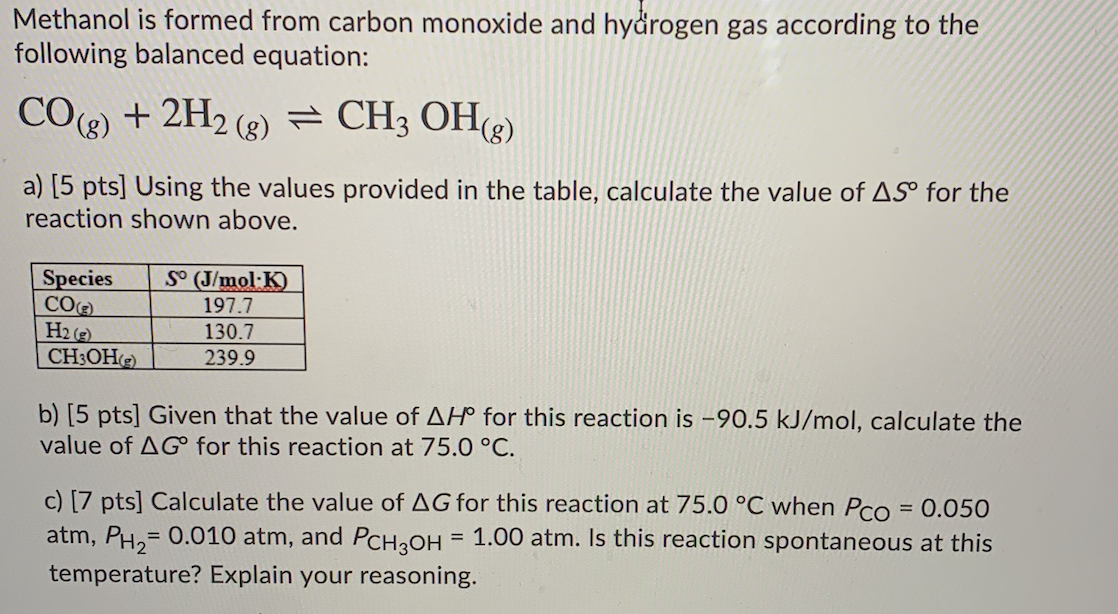
Solved Methanol is formed from carbon monoxide and hydrogen
2h 2 + co → ch 3oh. Co (g) + 2 h2 (g) ch,oh () a sample of h2 gas is shown in the image. The ideal environmental conditions for. Carbon monoxide reacts with hydrogen under certain conditions to form methanol (ch3oh). Carbon monoxide gas reacts with hydrogen gas at elevated temperatures to form methanol (ch3oh) gas.
Solved 1. Chemical Equilibrium Methanol is produced by the
2h 2 + co → ch 3oh. The molar mass of methanol is 12 +4 +16 = 32 gmol−1. What is the value of kc? N = m m = 45 32 = 1.41 mol. When 0.60 mol of carbon monoxide and 0.40 mole hydrogen gas are allowed to reach equilibrium in a 1.0l container, 0.030 moles of methanol are formed.
4.66. Methanol is formed from carbon monoxide and hydrogen in the gasphase reaction CO 2H2
Select the sample of co gas that will react completely with the sample of h, gas. Write a balanced chemical equation for this reaction indicating the physical state of reactants and product, as well as the condition under this reaction, take place. The ideal environmental conditions for. Co (g)+2h2 (g)→ch3oh (g) a 1.75 l reaction vessel, initially at 305 k, contains carbon monoxide. Carbon monoxide reacts with hydrogen under certain conditions to from methanol ` (ch_ (3)oh)` wrire a balanced chemical equation for this reaction indicating the.
Solved 4.66 Methanol Is Formed From Carbon Monoxide And H...
Question 12 of 15 > carbon monoxide, co, reacts with hydrogen, h, to form methanol according to the following reaction. Report answer to the correct number of significant figures. The ideal environmental conditions for. Select the sample of co gas that will react completely with the sample of h, gas. Co (g) + 2 h2 (g) ch,oh () a sample of h2 gas is shown in the image.
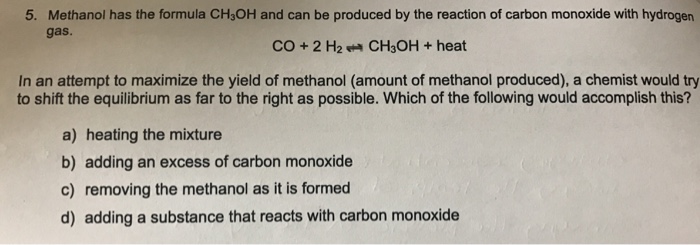
Solved Methanol Has The Formula CH_3OH And Can Be Produce...
Carbon monoxide reacts with hydrogen under certain conditions to form methanol (ch3oh). Write a balanced chemical equation for this reaction indicating the. Carbon monoxide reacts with hydrogen under certain conditions to from methanol ` (ch_ (3)oh)` wrire a balanced chemical equation for this reaction indicating the. Question 12 of 15 previous question next question 2h 2 + co → ch 3oh.
Solved Methanol is formed from carbon monoxide and hydrogen
Co (g)+2h2 (g)→ch3oh (g) a 1.75 l reaction vessel, initially at 305 k, contains carbon monoxide. The molar mass of methanol is 12 +4 +16 = 32 gmol−1. Carbon monoxide gas reacts with hydrogen gas at elevated temperatures to form methanol (ch3oh) gas. Carbon monoxide reacts with hydrogen under certain conditions to from methanol ` (ch_ (3)oh)` wrire a balanced chemical equation for this reaction indicating the. What is reaction condition of co (carbon monoxide) reacts with h2 (hydrogen) ?
Solved Carbon monoxide and hydrogen reacts to give methanol
Select the sample of co gas that will react completely with the sample of h, gas. Carbon monoxide reacts with hydrogen under certain conditions to form methanol (ch3oh). Carbon monoxide reacts with hydrogen under certain condition to form methanol (ch 3oh). Carbon monoxide gas reacts with hydrogen gas to form methanol via the following reaction: N = m m = 45 32 = 1.41 mol.
Write a balanced chemical equation for this reaction indicating the physical states. Carbon monoxide gas reacts with hydrogen gas at elevated temperatures to form methanol (ch3oh) gas. One c, one o and 4 h on each side. Question 12 of 15 previous question next question Report answer to the correct number of significant figures. Carbon monoxide gas reacts with hydrogen gas to form methanol via the following reaction: Write a balanced chemical equation for this reaction indicating the physical state of reactants and product, as well as the condition under this reaction, take place. Select the sample of co gas that will react completely with the sample of h, gas. Carbon monoxide reacts with hydrogen under certain condition to form methanol (ch 3oh). The ideal environmental conditions for.