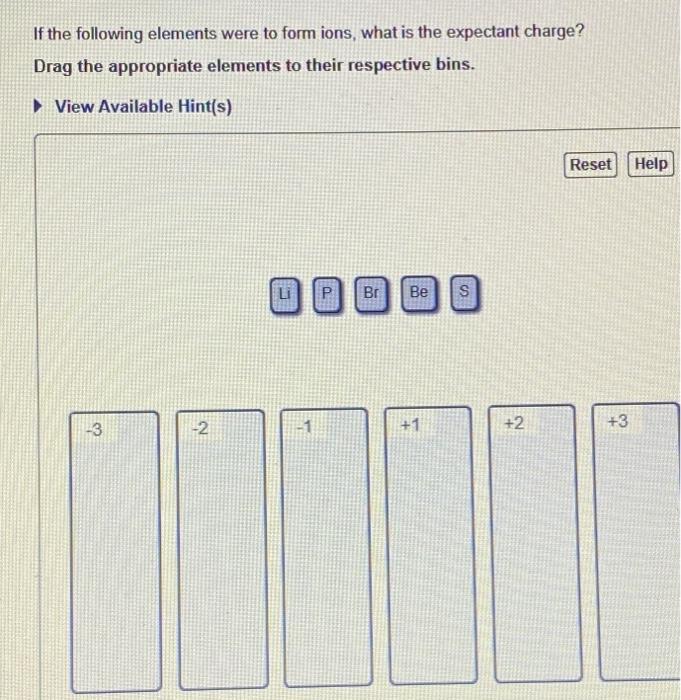
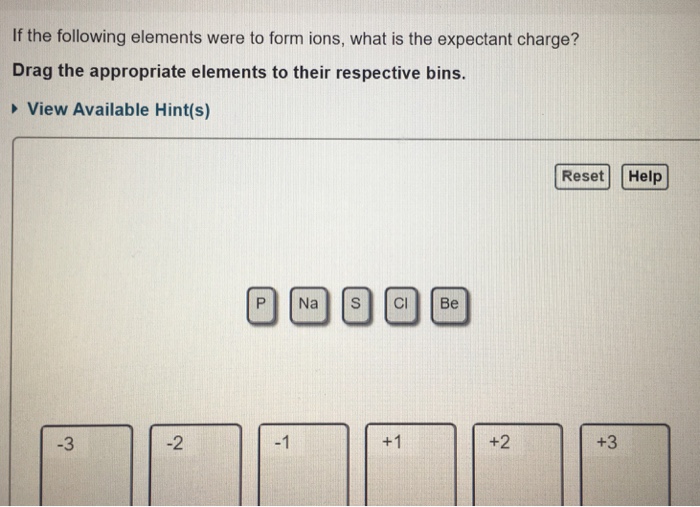
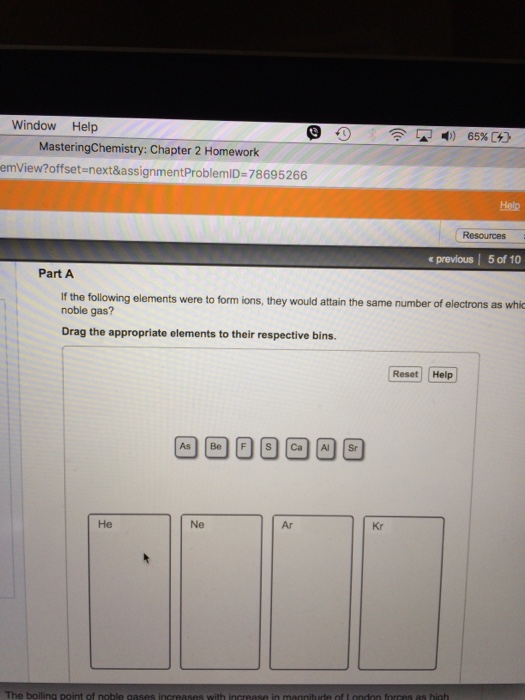
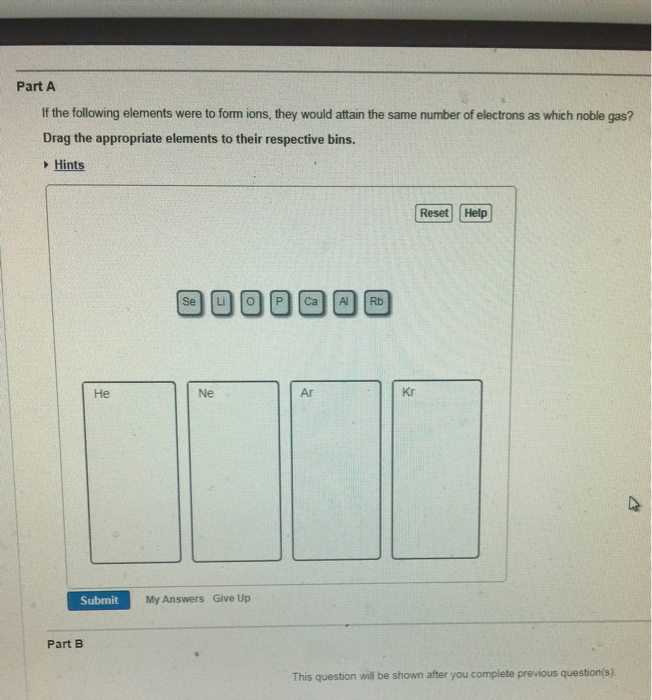
If The Following Elements Were To Form Ions
If the following elements were to form ions - Web ions and the periodic table (part a) if the following elements were to form ions, they would attain the same number of electrons as which noble gas? So arson is given arsenic, lithium offseason, phosphorus calcium and this one is sodium and rubidium argument. If the following elements were to form ions, what is the expectant charge? Atoms of elements lose or gain electrons to attain a stable. Web if the following elements were to form ions, they would attain the same number of electrons as which noble gas? There are two types of ions. + view available hint (s) reset help o. The naming conventions are listed in the. Drag the appropriate elements to their respective bins. Drag the appropriate elements to their respective bins. If the following elements were to form ions, what is the expectant charge? So if they form ions to which of. The ions as3− and sr+ each have 36 electrons like kr. If the following elements were to form ions, what is the expectant charge? Once the cation is named, the anion is then named.
Chemistry Archive February 01, 2016
Li → li⁺ + 1e⁻ be → be²⁺ + 2e⁻ while oxygen, nitrogen and. If the following elements were to form ions, what is the expectant charge? If the following elements were to form ions, they would attain the same number of electrons as which noble gas shose the appropriate elements to their. There are two types of ions. So if they form ions to which of.
Solved If the following elements were to form ions, what is
There are two types of ions. Web ions and the periodic table (part a) if the following elements were to form ions, they would attain the same number of electrons as which noble gas? If the following elements were to form ions, they would attain the same number of electrons as which noble gas shose the appropriate elements to their. The naming conventions are listed in the. If the following elements were to form ions, they would attain the same number of electrons as which noble gas shose the appropriate elements to their respective bins.
Solved If the following elements were to form ions, they
If the following elements were to form ions, what is the expectant charge? Web the s2− and ca2+ each has 18 electrons like ar. Ions are formed when an atom lose or gain the electrons. The naming conventions are listed in the. So arson is given arsenic, lithium offseason, phosphorus calcium and this one is sodium and rubidium argument.
Solved If The Following Elements Were To Form Ions, What
Se, be, n, ci, sc, na, y Atoms of elements lose or gain electrons to attain a stable. Web the s2− and ca2+ each has 18 electrons like ar. Drag the appropriate elements to their respective bins. Web in given list of elements li and be form positive ions because they easily lose their valance electrons.
Solved A. If The Following Elements Were To Form An Ionic...
Drag the appropriate elements to their respective bins. Web if an element gain electrons it will have an excess of negative charge (because the number of protons has not changed), forming what is known as an anion (a. So if they form ions to which of. Web ions and the periodic table (part a) if the following elements were to form ions, they would attain the same number of electrons as which noble gas? Drag the appropriate elements to their respective bins.
Solved If The Following Elements Were To Form An Ionic Co...
Once the cation is named, the anion is then named. Web the s2− and ca2+ each has 18 electrons like ar. Ions are formed when an atom lose or gain the electrons. Web in given list of elements li and be form positive ions because they easily lose their valance electrons. Li → li⁺ + 1e⁻ be → be²⁺ + 2e⁻ while oxygen, nitrogen and.
Solved If the following elements were to form ions, they
Li → li⁺ + 1e⁻ be → be²⁺ + 2e⁻ while oxygen, nitrogen and. Web ionic compounds are named according to their cation first, followed by their anion. The naming conventions are listed in the. Web if the following elements were to form ions, they would attain the same number of electrons as which noble gas? In your list that is o or po if the element can accomodate 12 electrons then it can have an expanded octet.
Solved Predict The Charge Of The Ion Formed By Each Of Th...
Drag the appropriate elements to their respective bins. If the following elements were to form ions, what is the expectant charge? Se, be, n, ci, sc, na, y Drag the appropriate elements to their respective bins. If the following elements were to form ions, what is the expectant charge?
Solved Part A If The Following Elements Were To Form Ions...
If the following elements were to form ions, what is the expectant charge? Li → li⁺ + 1e⁻ be → be²⁺ + 2e⁻ while oxygen, nitrogen and. Web ions and the periodic table (part a) if the following elements were to form ions, they would attain the same number of electrons as which noble gas? Once the cation is named, the anion is then named. Web the elements that form anions will have the electronic configuration similar to the noble gas that is in their period and those that form cations will have a.
Solved Part A If the following elements were to form ions,
In your list that is o or po if the element can accomodate 12 electrons then it can have an expanded octet. If the following elements were to form ions, what is the expectant charge? The naming conventions are listed in the. Li → li⁺ + 1e⁻ be → be²⁺ + 2e⁻ while oxygen, nitrogen and. Web if an element gain electrons it will have an excess of negative charge (because the number of protons has not changed), forming what is known as an anion (a.
So if they form ions to which of. Drag the appropriate elements to their respective bins. Web if the following elements were to form ions, they would attain the same number of electrons as which noble gas? Atoms of elements lose or gain electrons to attain a stable. Ions are formed when an atom lose or gain the electrons. Web ionic compounds are named according to their cation first, followed by their anion. If the following elements were to form ions, what is the expectant charge? There are two types of ions. Drag the appropriate elements to their respective bins. In your list that is o or po if the element can accomodate 12 electrons then it can have an expanded octet.