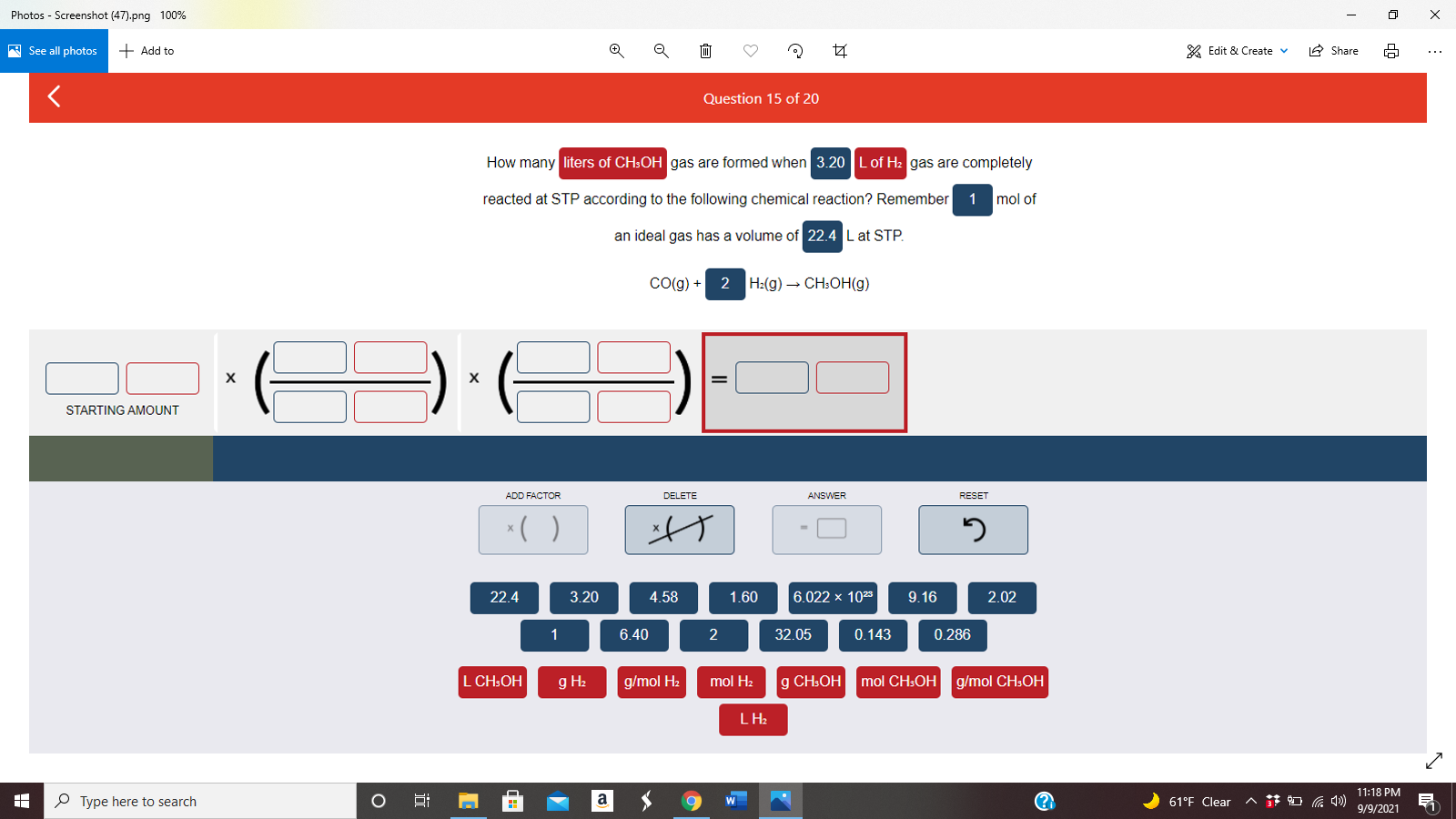
How Many Liters Of Ch3oh Gas Are Formed When 3 20
How many liters of ch3oh gas are formed when 3 20 - Remember 1 mol of an ideal gas has a volume of 22.4 l at stp. One mole oh ch three oh itch we have key points 20 h two so two moles h two producers, one mole of ch three oh itch morales, both h two produce is equals to three point. Remember 1 mol of an ideal gas has a volume of 22.4 l at. What will be its volume in liters if it is driven up into the mountains where atmospheric pressure is 0.868 atm? Co (g)+h2 (g) = ch3oh (g) question: Liters of ch₃oh gas are formed when 3.20 l of h₂ gas are completely reacted at stp according to the following chemical reaction? Remember 1 mol of an ideal gas has a volume of 22.4 lat stp co (g) + h2 (g) → ch;oh (g) 3.20 l h2 starting amount 3.20 l h2 add factor answer reset * ( ) 1.60 9.16 0.143. 3.20 l h2 x (1 mol h2/22.4 l h2) x (1 mol ch3oh/2 mol h2) x (22.4 l ch3oh/1 mol ch3oh) = 1.60 lch3oh answer from: How many liters of ch3oh gas are formed when 3.20 l of h2 gas are completely reacted at stp according to the following chemical reaction?. Quest show answer geosphere and. Co (g) + 2 h2 (g) → ch3oh (g) х starting amount add factor answer reset. Co(g)+2h2(g)→ch3oh(g) 1.what volume of h2 gas (in l), measured at 748 mmhg and 86 ∘c, is required to synthesize 26.0 g ch3oh?. How many liters of ch3oh gas are formed. 22.4 6.022 x 1023 6.40 0.286 3.20 9.16 2 n 4.58 2.02 32.05 1.60 1 0.143. Ch3oh can be synthesized by the following reaction.
Solved How Many Liters Of CH3OH Gas Are Formed When 3.20
One mole oh ch three oh itch we have key points 20 h two so two moles h two producers, one mole of ch three oh itch morales, both h two produce is equals to three point. Co (g)+h2 (g) = ch3oh (g) question: Co(g)+2h2(g)→ch3oh(g) 1.what volume of h2 gas (in l), measured at 748 mmhg and 86 ∘c, is required to synthesize 26.0 g ch3oh?. Ch3oh can be synthesized by the following reaction. Remember 1 mol of an ideal gas has a volume of 22.4 l at stp.
Solved Question 18 of 40 Submit How many liters of CH3OH gas
Co(g)+2h2(g)→ch3oh(g) 1.what volume of h2 gas (in l), measured at 748 mmhg and 86 ∘c, is required to synthesize 26.0 g ch3oh?. Co (g) + 2 h2 (g) → ch3oh (g) х starting amount add factor answer reset. How many liters of ch3oh gas are formed. Ch3oh can be synthesized by the following reaction. 7.49l 15.0 l of an ideal gas at 298 k and 3.36 atm are heated to 383 k with a new.
Solved How Many Liters Of CH3OH Gas Are Formed When 3.20
One mole oh ch three oh itch we have key points 20 h two so two moles h two producers, one mole of ch three oh itch morales, both h two produce is equals to three point. Remember 1 mol of an ideal gas has a volume of 22.4 l at stp. Remember 1 mol of an ideal gas has a volume of 22.4 l at. Co (g) + 2 h2 (g) → ch3oh (g) х starting amount add factor answer reset. How many liters of ch3oh gas are formed.
[Solved] How many liters of CH, OH gas are formed when 3.20 L of H2 gas are completely reacted
Co (g) + 2 h2 (g) → ch3oh (g) х starting amount add factor answer reset. 22.4 6.022 x 1023 6.40 0.286 3.20 9.16 2 n 4.58 2.02 32.05 1.60 1 0.143. Co(g)+2h2(g)→ch3oh(g) 1.what volume of h2 gas (in l), measured at 748 mmhg and 86 ∘c, is required to synthesize 26.0 g ch3oh?. Co (g)+h2 (g) = ch3oh (g) question: Remember 1 mol of an ideal gas has a volume of 22.4 l at stp.
Solved how many liters of CH3OH gas are formed when 3.20 L
What will be its volume in liters if it is driven up into the mountains where atmospheric pressure is 0.868 atm? 3.20 l h2 x (1 mol h2/22.4 l h2) x (1 mol ch3oh/2 mol h2) x (22.4 l ch3oh/1 mol ch3oh) = 1.60 lch3oh answer from: Remember 1 mol of an ideal gas has a volume of 22.4 l at. Co(g)+2h2(g)→ch3oh(g) 1.what volume of h2 gas (in l), measured at 748 mmhg and 86 ∘c, is required to synthesize 26.0 g ch3oh?. Co (g) + 2 h2 (g) → ch3oh (g) х starting amount add factor answer reset.
[Solved] How many liters of CH, OH gas are formed when 3.20 L of H2 gas are completely reacted
How many liters of ch3oh gas are formed. Remember 1 mol of an ideal gas has a volume of 22.4 l at. Ch3oh can be synthesized by the following reaction. Remember 1 mol of an ideal gas has a volume of 22.4 lat stp co (g) + h2 (g) → ch;oh (g) 3.20 l h2 starting amount 3.20 l h2 add factor answer reset * ( ) 1.60 9.16 0.143. Remember 1 mol of an ideal gas has a volume of 22.4 l at stp.
How many liters of CH3OH gas are formed when 3.20 L of H2 gas are completely reacted at STP
What will be its volume in liters if it is driven up into the mountains where atmospheric pressure is 0.868 atm? 7.49l 15.0 l of an ideal gas at 298 k and 3.36 atm are heated to 383 k with a new. Liters of ch₃oh gas are formed when 3.20 l of h₂ gas are completely reacted at stp according to the following chemical reaction? One mole oh ch three oh itch we have key points 20 h two so two moles h two producers, one mole of ch three oh itch morales, both h two produce is equals to three point. Remember 1 mol of an ideal gas has a volume of 22.4 lat stp co (g) + h2 (g) → ch;oh (g) 3.20 l h2 starting amount 3.20 l h2 add factor answer reset * ( ) 1.60 9.16 0.143.
[Solved] How many liters of CH, OH gas are formed when 3.20 L of H2 gas are completely reacted
7.49l 15.0 l of an ideal gas at 298 k and 3.36 atm are heated to 383 k with a new. 22.4 6.022 x 1023 6.40 0.286 3.20 9.16 2 n 4.58 2.02 32.05 1.60 1 0.143. Co(g)+2h2(g)→ch3oh(g) 1.what volume of h2 gas (in l), measured at 748 mmhg and 86 ∘c, is required to synthesize 26.0 g ch3oh?. What will be its volume in liters if it is driven up into the mountains where atmospheric pressure is 0.868 atm? Liters of ch₃oh gas are formed when 3.20 l of h₂ gas are completely reacted at stp according to the following chemical reaction?
How many liters of CH3OH gas are formed when 3.20 L are completely reacted at STP... HomeworkLib
Remember 1 mol of an ideal gas has a volume of 22.4 l at. Co (g)+h2 (g) = ch3oh (g) question: Quest show answer geosphere and. 22.4 6.022 x 1023 6.40 0.286 3.20 9.16 2 n 4.58 2.02 32.05 1.60 1 0.143. Liters of ch₃oh gas are formed when 3.20 l of h₂ gas are completely reacted at stp according to the following chemical reaction?
PPT Oxygen is 101 kPa; hydrogen is 505 kPa Oxygen is 202 kPa; hydrogen is 505 kPa PowerPoint
Co (g) + 2 h2 (g) → ch3oh (g) х starting amount add factor answer reset. Remember 1 mol of an ideal gas has a volume of 22.4 l at. Co(g)+2h2(g)→ch3oh(g) 1.what volume of h2 gas (in l), measured at 748 mmhg and 86 ∘c, is required to synthesize 26.0 g ch3oh?. Remember 1 mol of an ideal gas has a volume of 22.4 lat stp co (g) + h2 (g) → ch;oh (g) 3.20 l h2 starting amount 3.20 l h2 add factor answer reset * ( ) 1.60 9.16 0.143. How many liters of ch3oh gas are formed when 3.20 l of h2 gas are completely reacted at stp according to the following chemical reaction?.
How many liters of ch3oh gas are formed. What will be its volume in liters if it is driven up into the mountains where atmospheric pressure is 0.868 atm? Remember 1 mol of an ideal gas has a volume of 22.4 lat stp co (g) + h2 (g) → ch;oh (g) 3.20 l h2 starting amount 3.20 l h2 add factor answer reset * ( ) 1.60 9.16 0.143. Remember 1 mol of an ideal gas has a volume of 22.4 l at stp. Quest show answer geosphere and. How many liters of ch3oh gas are formed when 3.20 l of h2 gas are completely reacted at stp according to the following chemical reaction?. 3.20 l h2 x (1 mol h2/22.4 l h2) x (1 mol ch3oh/2 mol h2) x (22.4 l ch3oh/1 mol ch3oh) = 1.60 lch3oh answer from: Ch3oh can be synthesized by the following reaction. Co(g)+2h2(g)→ch3oh(g) 1.what volume of h2 gas (in l), measured at 748 mmhg and 86 ∘c, is required to synthesize 26.0 g ch3oh?. 22.4 6.022 x 1023 6.40 0.286 3.20 9.16 2 n 4.58 2.02 32.05 1.60 1 0.143.