Do Calcium And Rubidium Form An Ionic Compound
Do calcium and rubidium form an ionic compound - Web yes it is an ionic compound. Thus, in $rbcl$, rubidium forms a cation and chlorine forms an anion and an ionic bond is formed. Click the card to flip 👆. Web rubidium metal on loss of one electron attains noble gas like configuration and chlorine and bromine can gain an electron each t=and achieve noble gas like configuration. Web rubidium an alkali metal does not form compounds or ionic bonds with calcium an alkaline earth metal. The lattice is described as follows: Web li 2 cr 2 o 7. Rubidium forms two other oxides (rb2o and rb2o3). Web rubidium metal on loss of one electron attains noble gas like configuration and chlorine and bromine can gain an electron each t=and achieve noble gas like configuration. What is the ionic compound of rubidium iodide? Molecular compounds are electrically neutral. Rubidium iodide is a salt with. Thus, in $rbcl$, rubidium forms a cation and chlorine forms an anion and an ionic bond is formed. Web when calcium reacts with tellurium to form an ionic compound each metal atom loses electrons and each nonmetal atom gains electrons. The two each form compounds with several of the same elements (e.g.
Do Calcium and Rubidium Form an Ionic Compound KasenhasLopez
They are formed by the donation of one. Web li 2 cr 2 o 7. In compounds of rubidium (where known), the most. Question 7 (1 point) calcium, rubidium, and fluorine form an ionic compound which crystallizes in a cubic structure. The two each form compounds with several of the same elements (e.g.
Do Calcium and Rubidium Form an Ionic Compound
Web 1925 rows rubidium nitrite: Thus, in $rbcl$, rubidium forms a cation and chlorine forms an anion and an ionic bond is formed. Web rubidium (an alkali metal) does not form compounds or ionic bonds with calcium (an alkaline earth metal). Web yes it is an ionic compound. The lattice is described as follows:
Do Calcium and Rubidium Form an Ionic Compound KasenhasLopez
Web rubidium an alkali metal does not form compounds or ionic bonds with calcium an alkaline earth metal. The two each form compounds with several of the same elements (e.g. What is the ionic compound of rubidium iodide? Web 1925 rows rubidium nitrite: Web rubidium metal on loss of one electron attains noble gas like configuration and chlorine and bromine can gain an electron each t=and achieve noble gas like configuration.
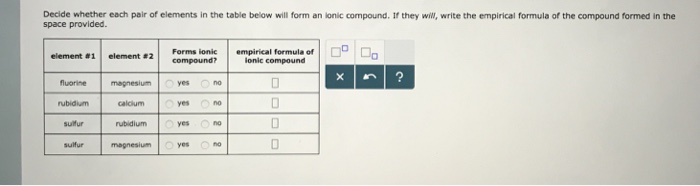
Solved Decide whether each pair of elements in the table
Web rubidium (an alkali metal) does not form compounds or ionic bonds with calcium (an alkaline earth metal). What is the ionic compound of rubidium iodide? Web rubidium metal on loss of one electron attains noble gas like configuration and chlorine and bromine can gain an electron each t=and achieve noble gas like configuration. Rubidium forms two other oxides (rb2o and rb2o3). Molecular compounds are electrically neutral.
Solved Decide Whether Each Pair Of Elements In The Table
Web 1925 rows rubidium nitrite: The lattice is described as follows: Web when calcium reacts with tellurium to form an ionic compound each metal atom loses electrons and each nonmetal atom gains electrons. The subscript for both calcium and oxygen is 1. What is the ionic compound of rubidium iodide?
Gcse c4 chemical patterns bonding & periodic table revision
Web rubidium (an alkali metal) does not form compounds or ionic bonds with calcium (an alkaline earth metal). Web rubidium metal on loss of one electron attains noble gas like configuration and chlorine and bromine can gain an electron each t=and achieve noble gas like configuration. Web li 2 cr 2 o 7. Web covalent or molecular compounds form when elements share electrons in a covalent bond to form molecules. Rubidium iodide is a salt with.
PPT Chapter 9 Naming Compounds and Writing Formulas PowerPoint Presentation ID3645014
The lattice is described as follows: Rubidium iodide is a salt with. Thus, in $rbcl$, rubidium forms a cation and chlorine forms an anion and an ionic bond is formed. Web when calcium reacts with tellurium to form an ionic compound each metal atom loses electrons and each nonmetal atom gains electrons. Web rubidium metal on loss of one electron attains noble gas like configuration and chlorine and bromine can gain an electron each t=and achieve noble gas like configuration.
NWTC General Chemistry Ch 06
Question 7 (1 point) calcium, rubidium, and fluorine form an ionic compound which crystallizes in a cubic structure. Web rubidium metal on loss of one electron attains noble gas like configuration and chlorine and bromine can gain an electron each t=and achieve noble gas like configuration. Web when calcium reacts with tellurium to form an ionic compound each metal atom loses electrons and each nonmetal atom gains electrons. They are formed by the donation of one. In compounds of rubidium (where known), the most.
Solved Decide whether each pair of elements in the table
Web rubidium metal on loss of one electron attains noble gas like configuration and chlorine and bromine can gain an electron each t=and achieve noble gas like configuration. The two each form compounds with several of the same elements (e.g. Web rubidium peroxides (rb2o2) can be formed by oxidation of the metal with the required amount of oxygen. The subscript for both calcium and oxygen is 1. Web rubidium an alkali metal does not form compounds or ionic bonds with calcium an alkaline earth metal.
Chapter 2
Web covalent or molecular compounds form when elements share electrons in a covalent bond to form molecules. What is the ionic compound of rubidium iodide? In compounds of rubidium (where known), the most. Web rubidium peroxides (rb2o2) can be formed by oxidation of the metal with the required amount of oxygen. Web rubidium (an alkali metal) does not form compounds or ionic bonds with calcium (an alkaline earth metal).
Web rubidium an alkali metal does not form compounds or ionic bonds with calcium an alkaline earth metal. What is the ionic compound of rubidium iodide? Question 7 (1 point) calcium, rubidium, and fluorine form an ionic compound which crystallizes in a cubic structure. The subscript for both calcium and oxygen is 1. The lattice is described as follows: Web when calcium reacts with tellurium to form an ionic compound each metal atom loses electrons and each nonmetal atom gains electrons. Thus, in $rbcl$, rubidium forms a cation and chlorine forms an anion and an ionic bond is formed. Web 1925 rows rubidium nitrite: Web rubidium peroxides (rb2o2) can be formed by oxidation of the metal with the required amount of oxygen. Web li 2 cr 2 o 7.